allergies, allergy, allergy shots, allergy symptoms, Asthma, coronavirus, Dr. Marc Goldstein, medicine
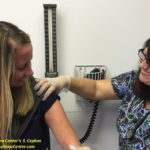
Third mRNA COVID-19 Booster Shot Vaccination for Immunocompromised Individuals
About 2.7% of all American adults are considered to be immunocompromised, which puts them at a higher risk of having a severe COVID-19 infection and spreading the virus to others. There are now several studies that have shown that moderately to severely immunocompromised individuals have higher risk of developing severe COVID-19 infection with prolonged illness and prolonged viral shedding. Other evidence suggests that such individuals may also produce lower levels of antibody protection after receiving 2 doses of COVID-19 mRNA vaccine (Pfizer, Moderna) compared to individuals who have normal immune systems. Immunocompromised individuals will therefore experience slower protective vaccine effects as well as have a higher rate of hospitalization due to breakthrough infections.
Has the Third COVID-19 Booster Shot Been Approved for the Immunocompromised?
The Center for Diseases Control and Prevention (CDC) and FDA have endorsed an additional mRNA COVID-19 vaccine to patients who are moderately to severely immunocompromised and who have completed a 2-dose mRNA primary series with either Pfizer – BioNTech or Moderna vaccines. The additional mRNA COVID-19 vaccine must be provided at least 4 weeks (28 days) after the second dose of mRNA vaccine.
Who is an Immunocompromised Individual and Qualifies for Getting the COVID-19 Booster Vaccination?
Patients in our practice who are moderately to severely immunocompromised include those with moderate or severe primary or secondary immunodeficiencies and those individuals on immunosuppressive treatments. Immunodeficient patients with common variable hypogammaglobulinemia (CVID), selective IgA deficiency, or selective IgM immunodeficiency should get a third COVID-19 vaccination. In addition, patients who require high dose corticosteroids (greater than 20 mg per day) on a regular basis are also considered as being immunocompromised. Patients seen at The Asthma Center who may be immunocompromised due to treatment prescribed by another physician for organ transplant, CAR-T cell therapy or stem cell transplant, cancer chemotherapy or immunotherapy, autoimmune disease, HIV, or other immunosuppressive or immunomodulatory drugs (some biologics, antimetabolites, alkylating agents) should discuss with their Asthma Center physician as to whether they should get a third dose. Other than those patients who have moderate to severe immunocompromise, no other groups are eligible to receive an additional mRNA COVID-19 vaccine at this time. Additional doses of the COVID-19 vaccine should be with the same vaccine as the initial 2 dose mRNA vaccine but if it is not available, another product may be given.
What About J&J COVID-19 Booster Shot Vaccination for the Immunocompromised?
There is not enough evidence to support whether the use of additional mRNA COVID-19 vaccine dose should be given after a single dose of Johnson & Johnson/Janssen COVID-19 vaccine in immunocompromised people. The FDA has not approved the use of additional mRNA vaccine after a single dose of the Johnson & Johnson/Janssen COVID-19 vaccine series in any individuals. The recommendation of additional doses may change in the future and CDC and FDA updates will be provided as they become available.
Recent
Popular